Although the synthesis of α‑tertiary amino acids (ATAAs) has been extensively studied, the development of an inexpensive and facile methodology to incorporate multifunctionality on ATAAs remains challenging. The facile synthesis of unnatural ATAAs using enzymatic or transition metals catalysis (Strecker reactions) been extensively explored. However, those methods were applied to limited functional groups, toxic reagents, and multi-step protocols. Most recently, Easy to operate and green radical chemistry has utilized to novel unnatural ATAA libraries (Fig. 1a). Unfortunately, most of these methods suffer from the utilization of stoichiometric amounts of radical initiators, higher temperatures, and multi-step catalysts. An efficient and environmentally friendly method that allows streamlined access to functionalized ATAAs still needs to be discovered.
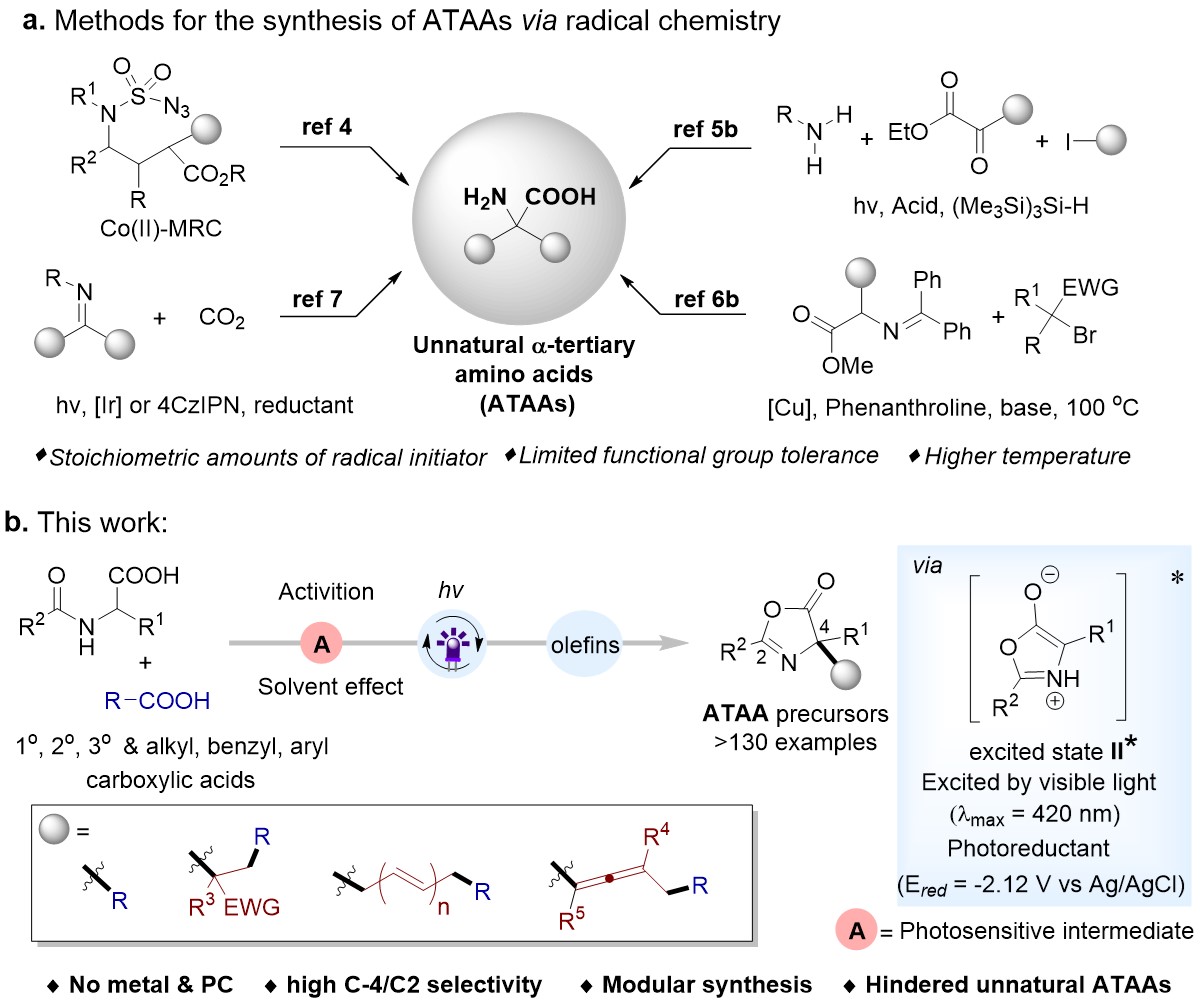
Figure 1
The C4 functionalization of oxazolones derived from amino acids is a common method for the synthesis of ATAAs, However, utilization of oxazolones hasn’t been explored very much in radical reaction. It is worth noting that compared with the widely studied nucleophilic reaction of oxazolones C4, the synthesis of ATAAs in the photocatalytic field using oxazolones as a radical precursor has not been reported. Basis of previous work, Professor Ke Zheng's team reported the first radical cross-coupling reaction of C(sp3)-C(sp3) and C(sp3)-C(sp2) selectively under the excitation of visible light. In this method, the oxazolones were acted as radical precursor and efficient reductant in polar solvent. Various and complex (ATAAs) derivatives were obtained under mild conditions with an absence of metals, photocatalysts, and all other additives (Fig. 1b). We further demonstrated the potential of carrying out a facile one-pot reaction by directly using carboxylic acids or α-amino acids as substrates. Moreover, this is first time to modular construction of functionalized allenic ATAA derivatives by using radical-coupling reactions. Its excellent functional group tolerance, and its late-stage applicability make it promising for both academia and industry applications. This simple radical approach provides a less expensive, nontoxic alternative to classical photochemistry.
This research was recently published in Angew. Chem. Int. Ed. with the title “Modular Construction of Unnatural α-Tertiary Amino Acid Derivatives by Multicomponent Radical Cross-Couplings”, link: https://doi.org/10.1002/anie.202210755. This work is supported by the National Natural Science Foundation of China, Sichuan Science and Technology Program, and Sichuan University.
