RESEARCH INTERESTS:
(1) Synthesis of phosphorus ligand and its rhodium complex
(2) Hydroformylation of alkene and alkyne
(3) Electrochemical protocol for the construction of C-C(N) bond
EDUCATION AND ACADEMIC CAREER:
2011 to date, College of Chemistry, Sichuan University. Associate Professor
2009-2010, Complex Hydride Materials Research Group 1901, Dalian Institute Chemical Physics, ACS. Exchange student. Supervisor: Prof. Ping Chen & Hua Chen
2007-2009, Hydrogen Storage Group, Surface Science Lab, Dept. of Physics, National University of Singapore. Exchange student. Supervisor: Prof. Ping Chen & Hua Chen
2005-2007, Key Laboratory of Green Chemistry and Technology, Ministry of Education, Institute of Homogeneous Catalysis, College of Chemistry, Sichuan University, Master in Organic Chemistry, Supervisor: Prof. Hua Chen
2001-2005, College of Chemistry, Sichuan University, Bachelor in Organic Chemistry
HONORS AND AWARDS:
The first prize of the Second "Inquiry-based Small Class" Teaching Competition of Sichuan University
Sichuan University Outstanding Teacher Award
Excellent Communist Party member of Sichuan University
SELECTED PUBLICATIONS:
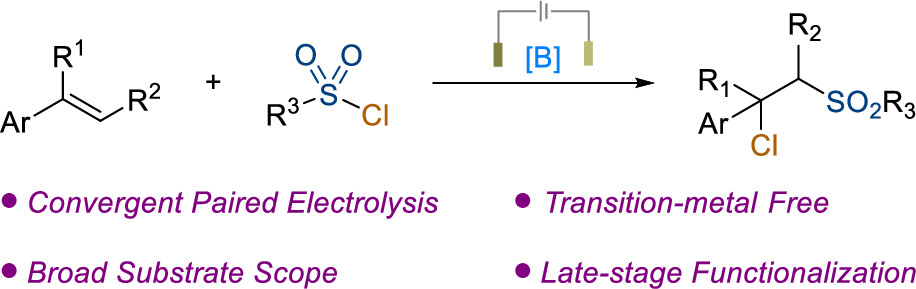
1. Peng, X.; Tao, S.; Xue, W.; Fu, H.; Xu, J.; Li, R.; Chen, H.; Zheng, X.* Organoboron-Promoted Electrochemical Chlorosulfonylation of Alkenes with Sulfonyl Chlorides, Org Lett 2024, 26, 4018-4023.
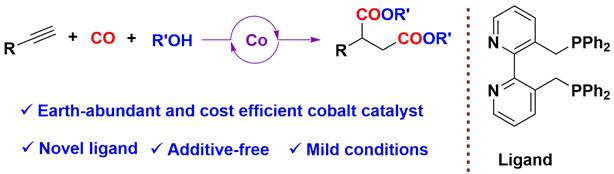
2. Luo, M.; Liu, Z.; Chen, H.; Fu, H.; Li, R.; Zheng, X.* Bifunctional diphosphine ligand-enabled cobalt catalyzed bis-alkoxycarbonylation of alkynes, J Catal 2024, 433, 115459.
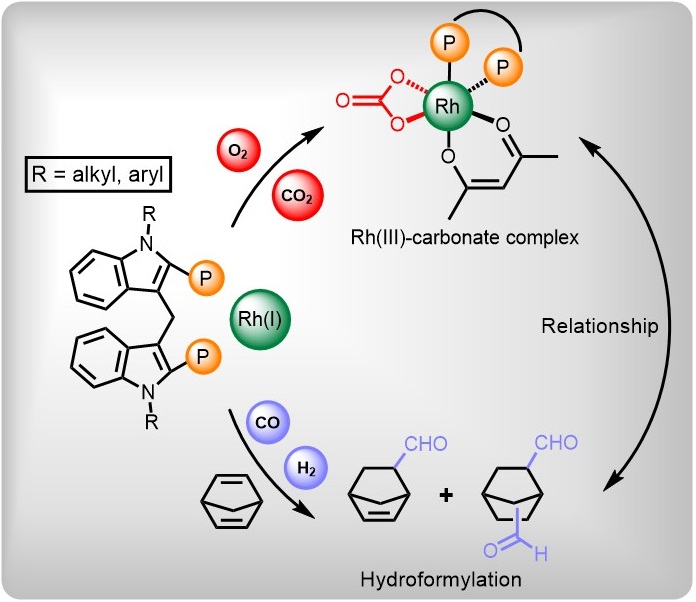
3. Liu, Z.; Zheng, X.;* Fu, H.; Yuan, M.; Li, R.; Chen, H.* Rhodium-catalyzed hydroformylation of strained dialkenes: Ligand effect on product selectivity and complex coordination mode, J. Catal. 2023, 422, 77.
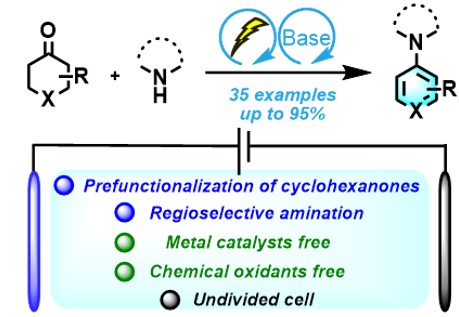
4. Tao, S. K.; Chen, S. Y.; Feng, M. L.; Xu, J. Q.; Yuan, M. L.; Fu, H. Y.; Li, R. X.; Chen, H.; Zheng, X. L.;* Yu, X. Q. Electrochemical Cross-Dehydrogenative Aromatization Protocol for the Synthesis of Aromatic Amines, Org Lett, 2022, 24, 1011-1016.
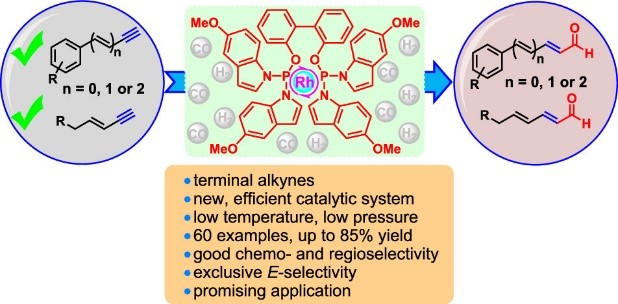
5. Zhao, J.#; Zheng, X. L.;# Tao, S.; Zhu, Y.; Yi, J.; Tang, S.; Li, R.; Chen, H.; Fu, H.; * Yuan, M.,* Selective Rhodium-Catalyzed Hydroformylation of Terminal Arylalkynes and Conjugated Enynes to (Poly)enals Enabled by a π Acceptor Biphosphoramidite Ligand. Org Lett 2021, 23, 6067.
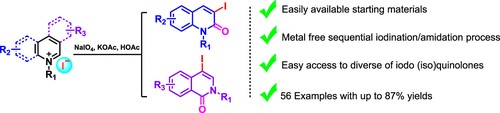
6. Tang, J.; Chen, X.; Zhao, C. Q.; Li, W. J.; Li, S.; Zheng, X. L.*; Yuan, M. L.; Fu, H. Y.*; Li, R. X.; Chen, H. Iodination/Amidation of the N-Alkyl (Iso)quinolinium Salts. J Org Chem 2021, 86, 716.
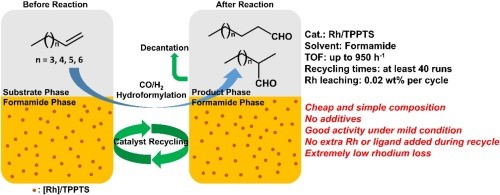
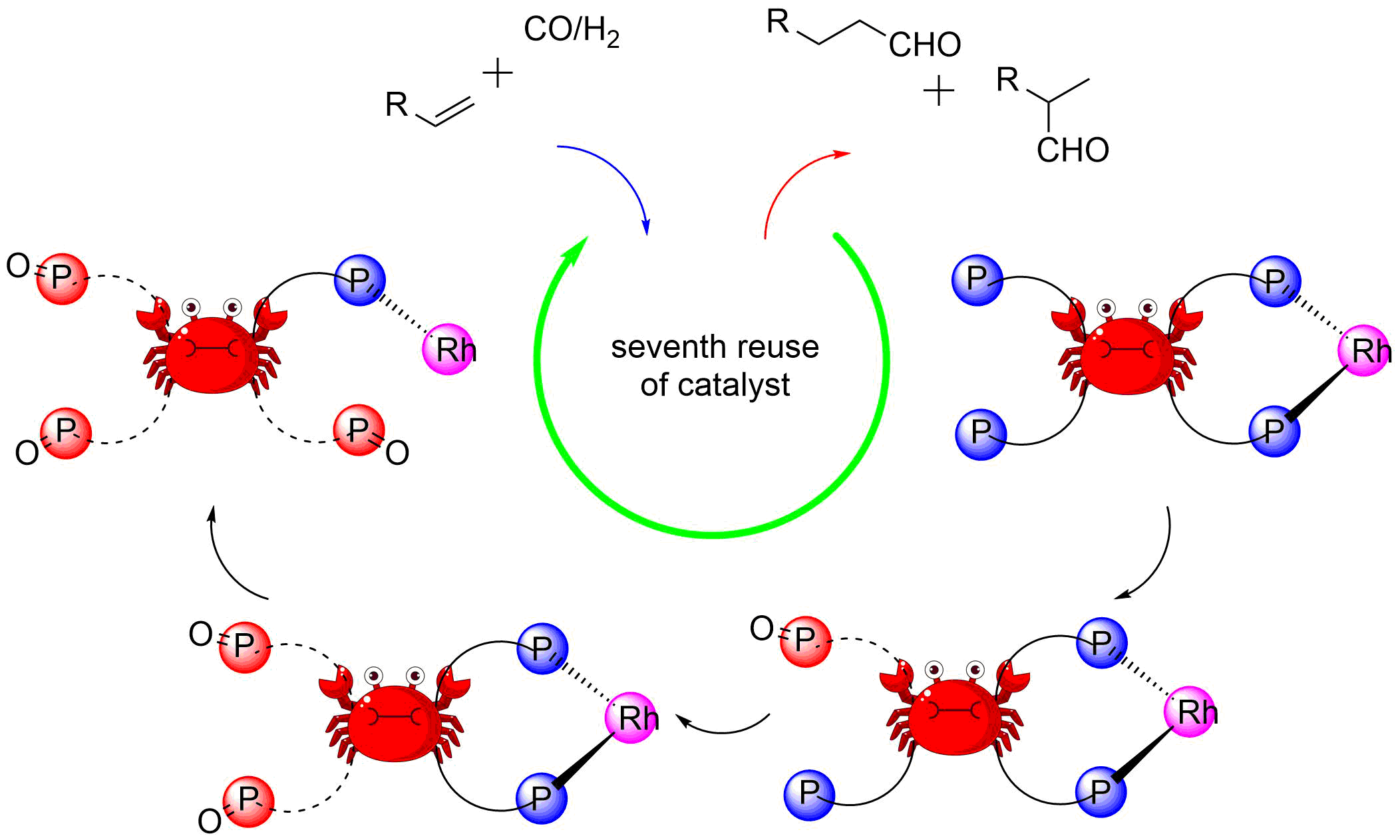
7. Yi, J.; Zhao, J.; Tang, S.; Yang, C.; Fu, H.; Zheng, X. L.*; Chen, H.*; Yuan, M.; Li, R. A novel biphasic and recyclable system based on formamide for the hydroformylation of long-chain alkenes with water-soluble phosphine rhodium catalyst. Mol Catal 2021, 505, 111502.
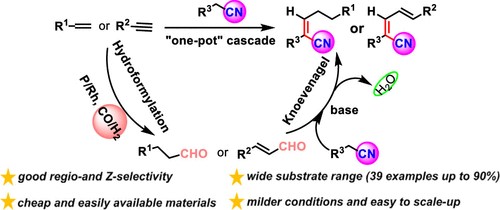
8. Jiang, Y.; Li, C.; Tang, S.; Tao, S.; Yuan, M.; Li, R.; Chen, H.; Fu, H.; Zheng, X.* Practical Synthesis of (Z)-α,β-Unsaturated Nitriles via a One-Pot Sequential Hydroformylation/Knoevenagel Reaction, J Org Chem, 2021, 86: 15413-15422.
9. Tang, S.; Jiang, Y.; Yi, J.; Duan, X.; Fu, H.; Li, R.; Yuan, M.; Chen, H.; Yang, C.; Zheng, X. Highly regioselective homogeneous isomerization-hydroformylation of 2-butene with water- and air-stable phosphoramidite bidentate ligand. Mol Catal 2021, 508, 111598.
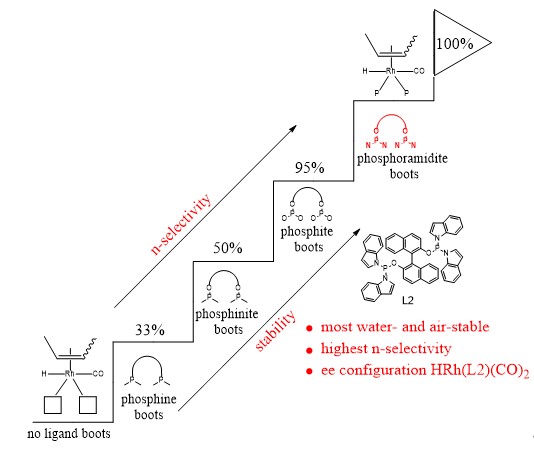
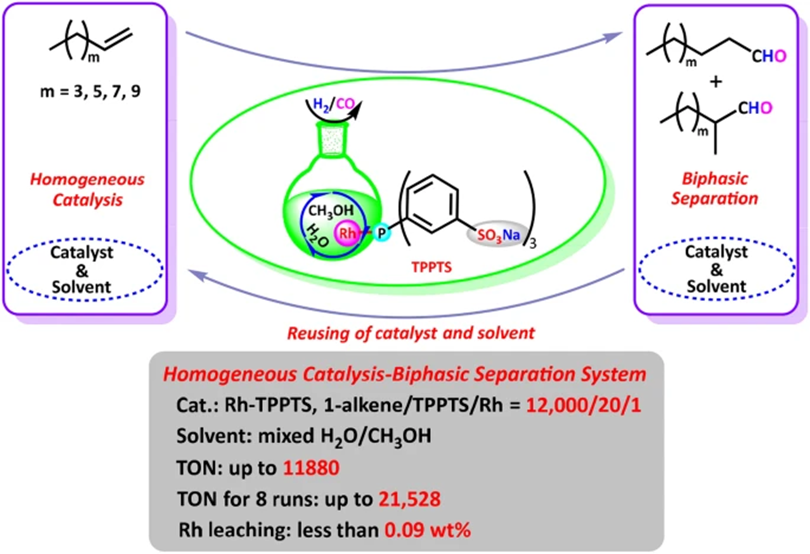
10. Zhao, J.; Yi, J.; Yang, C.; Wan, K.; Duan, X.; Tang, S.; Fu, H.; Zheng, X. L.*; Yuan, M.*; Li, R.; Chen, H. A Novel Strategy of Homogeneous Catalysis and Highly Efficient Recycling of Aqueous Catalyst for the hydroformylation of Higher Olefins Based on a Simple Methanol/Water Mixed Solvent. Catal Lett 2021,151, 1273–1281 .
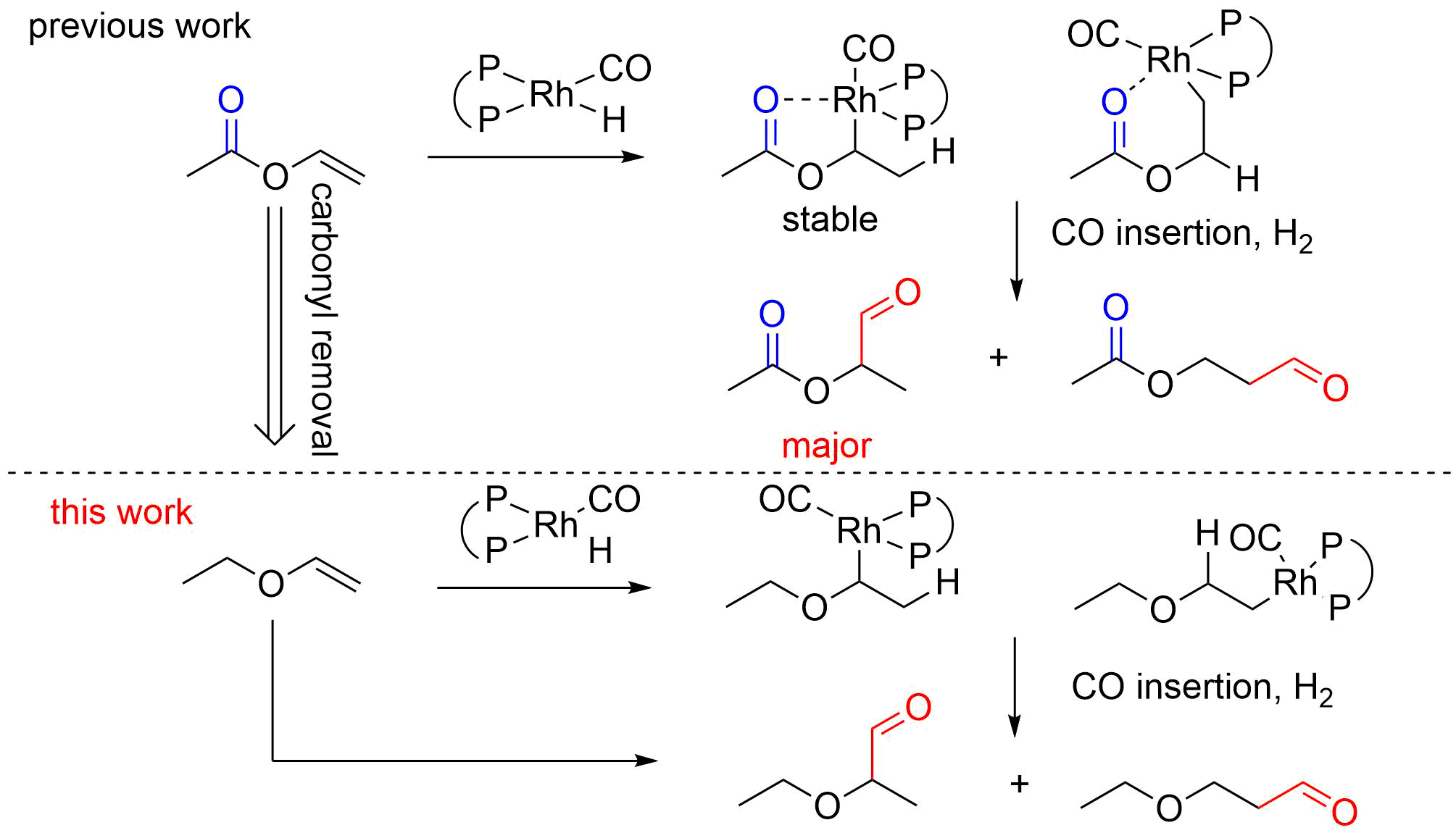
11. Wan, K.; Zhao, J.; Qin, S.; Zheng, X. L.*; Fu, H.; Li, R.; Chen, H.*; Yang, J.; Yang, C. Linear‐selective hydroformylation of vinyl ether using Rh (acac)(2,2′‐ bis{(di[1H‐indol‐1‐yl]phosphanyl)oxy}‐1,1′‐binaphthalene) – Possible way to synthesize 1,3‐propanediol. Applied Organometallic Chemistry, 2020, 34(10): e5863.
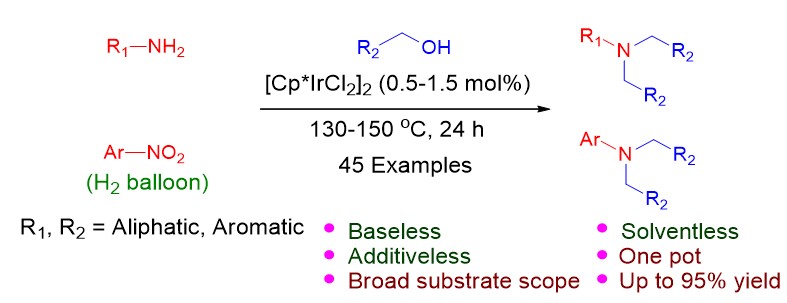
12. Li, C.; Wan, K. F.; Guo, F. Y.; Wu, Q. H.; Yuan, M. L.; Li, R. X.; Fu, H. Y.; Zheng, X. L.*; Chen, H.*, Iridium-Catalyzed Alkylation of Amine and Nitrobenzene with Alcohol to Tertiary Amine under Base- and Solvent-Free Conditions. J Org Chem 2019, 84, (4), 2158-2168.
13. Zhou, F.; Zhang, L.; Wu, Q.; Guo, F.; Tang, S.; Xu, B.; Yuan, M.; Fu, H.; Li, R.; Zheng, X. L.*, Chen, H.*, A new air-stable and reusable tetraphosphine ligand for rhodium-catalyzed hydroformylation of terminal olefins at low temperature. Appl Organometal Chem 2019, 33, (1), e4646.
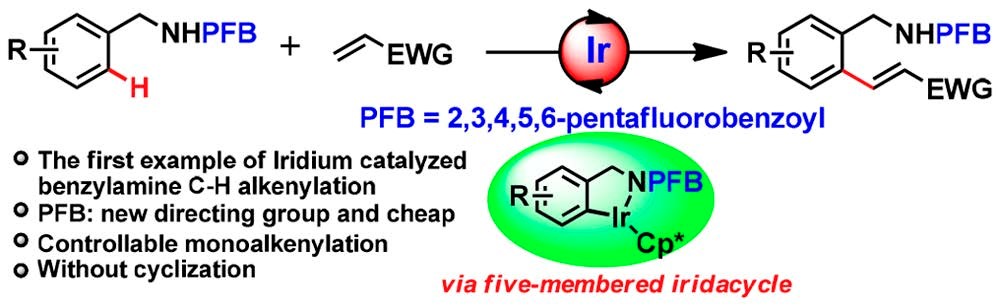
14. Yang, X.; Sun, R.; Zhang, C.; Zheng, X. L.*; Yuan, M.; Fu, H.*; Li, R.; Chen, H., Iridium-Catalyzed Benzylamine C-H Alkenylation Enabled by Pentafluorobenzoyl as the Directing Group. Org Lett 2019, 21, (4), 1002-1006.
15. Liu, Y.-l.; Zhao, J.-g.; Zhao, Y.-j.; Liu, H.-M.; Fu, H.-y.; Zheng, X. L.*; Yuan, M.-l.*; Li, R.-x.; Chen, H., Homogeneous hydroformylation of long chain alkenes catalyzed by water soluble phosphine rhodium complex in CH3OH and efficient catalyst cycling. RSA Adv 2019, 9, (13), 7382-7387.
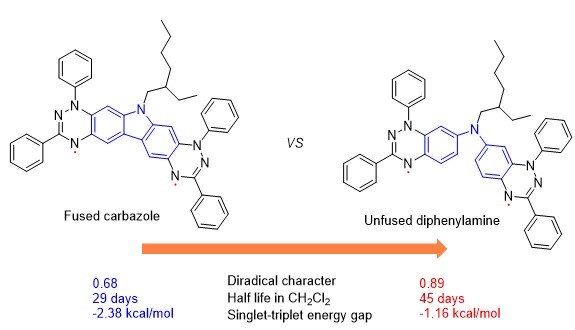
16. Hu, X.; Chen, H.; Zhao, L.; Miao, M.-s.; Zheng, X. L.*; Zheng, Y.*, Nitrogen-coupled blatter diradicals: the fused versus unfused bridges. J Mater Chem C 2019, 7, 10460-10464.
17. Zhao, Y.; Liu, Y.; Wei, J.; Fu, H.; Zheng, X. L.*; Yuan, M.*; Li, R.; Chen, H., Nonaqueous Biphasic Hydroformylation of Long Chain Alkenes Catalyzed by Water Soluble Phosphine Rhodium Catalyst with Polyethylene Glycol Instead of Water. Catal Lett 2017, 148, (1), 438-442.
18. Wu, Q.; Zhou, F.; Shu, X.; Jian, L.; Xu, B.; Zheng, X. L.*; Yuan, M.; Fu, H.; Li, R.; Chen, H.*, Synthesis and application of PNP pincer ligands in rhodium-catalyzed hydroformylation of cycloolefins. RSC Adv. 2016, 6, (109), 107305-107309.
19. Han, W.; Qin, S.; Shu, X.; Wu, Q.; Xu, B.; Li, R.; Zheng, X. L.*; Chen, H.*, Synthesis of phosphorus amidite ligand and investigation of its flexibility impact on rhodium-catalyzed hydroformylation of 1-octene. RSC Adv. 2016, 6, (58), 53012-53016.
20. Zheng, X. L.; Zheng, C.-Y.; Zhou, F.-D.; Fu, H.-Y.; Yuan, M.-L.; Li, R.-X.; Xu, B.; Chen, H., Highly active rhodium/phosphorus catalytic system for the hydroformylation of α-methylstyrene. Chin Chem Lett 2016, 27, (5), 678-680.
21. Zheng, X. L.; Chua, Y.; Xiong, Z.; Chen, W.; Jiang, Z.; Wu, G.; Chen, P., The effect of NH3 content on hydrogen release from LiBH4–NH3 system. Int J Hydrogen Energ 2015, 40, (13), 4573-4578.
22. Zheng, X. L.; Yang, Q.; Li, Z.; Zhu, Z.; Cui, X.; Fu, H.; Chen, H.; Li, R., Trans-chloro-(1-naphthyl)bis[tris-(4-methoxyphenyl)phosphane]-nickel(II) catalyzed Suzuki-Miyaura coupling of aryl chlorides with phenylboronic acid. Catal Commun 2014, 57, 143-147.
23. Du, Y. Zheng, X. L.*, Enantioselective Hydrogenation of Ethyl Pyruvate Catalyzed by 1,2-Diphenyl-ethylenediamine-Modified Iridium Complex: Effect of Solvent. Asian J Chem 2014, 26, 2.
24. Zheng, C.Y.; Mo, M.; Liang, H.R.; Zheng, X.L.; Fu, H.Y.; Yuan, M. L.; Li, R.X.; Chen, H. Rhodium/bisphosphite catalytic system for hydroformylation of styrene and its derivatives. Appl Organomet Chem 2013, 27, 474-478..
25. Zheng, X. L.; Wu, G. T.; He, T.; Chu, H. L.; Chen, H.; Chen, P., Improved hydrogen desorption properties of Co-doped Li2BNH6. Chin Sci Bull 2011, 56, (23), 2481-2485.
26. Zheng, X. L.; Wu, G. T.; Li, W.; Xiong, Z. T.; He, T.; Guo, J. P.; Chen, H.; Chen, P., Releasing 17.8 wt% H2 from lithium borohydride ammoniate. Energy Environ Sci 2011, 4, (9), 3593-3600.
27. Zheng, X. L.; Xiong, Z. T.; Lim, Y. H.; Wu, G. T.; Chen, P.; Chen, H., Improving Effects of LiH and Co-Catalyst on the Dehydrogenation of Li4BN3H10. J Phys Chem C 2011, 115, (17), 8840-8844.
28. Zheng, X. L.; Xu, W. L.; Xiong, Z. T.; Chua, Y. S.; Wu, G. T.; Qin, S.; Chen, H.; Chen, P., Ambient temperature hydrogen desorption from LiAlH4-LiNH2 mediated by HMPA. J Mater Chem 2009, 19, (44), 8426-8431.
29. Zheng, X. L.; Xiong, Z. T.; Qin, S.; Chua, Y. S.; Chen, H.; Chen, P., Dehydrogenation of LiAlH4 in HMPA. Int J Hydrogen Energ 2008, 33, (13), 3346-3350.
